States of Matter
Solids, Liquids, Gases, Plasma
In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Often the state of matter of a substance may be changed by adding or removing heat energy from it. For example, the addition of heat can melt ice into liquid water and turn water into steam.
Solids



examples of solids: wood, sand, mountains, ice (solid water), feather, brick, rock, banana, chair, gold
A solid has a definite shape and volume. In a solid, the particles (ions, atoms or molecules) are closely packed together. The forces between particles are strong so that the
particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when
broken or cut.
Liquids



examples of liquids: water, milk, oil, tea
A liquid has a definite volume, but takes the shape of its container. When a solid is heated above its melting point, it becomes liquid. Liquids are difficult to compress. When
you compress something, you take a certain amount of material and force it into a smaller space or volume. You force the atoms closer together. Most solids are very difficult
to compress while gases are easier. The highest temperature at which a given liquid can exist is its critical temperature.
Gases



examples of gases: air, water vapor, oxygen, helium, freon
The molecules in gases are really spread out, full of energy, and constantly moving around in random ways. A gas has neither a definite volume nor a definite shape. It is
compressible; not only will a gas conform to the shape of its container but it will also expand to fill the container. A liquid may be converted to a gas by heating at constant
pressure to the boiling point.
Plasma




examples of plasma: lightning, electric sparks, the nothern lights, fluorescent lights, neon lights, plasma televisions, some types of flame and stars
Like a gas, plasma does not have definite shape or volume. Unlike gases, plasmas are electrically conductive, produce magnetic fields and electric currents, and respond
strongly to electromagnetic forces. Plasma is made up of groups of positively and negatively charged particles. Plasma may be formed by heating and ionizing a gas.
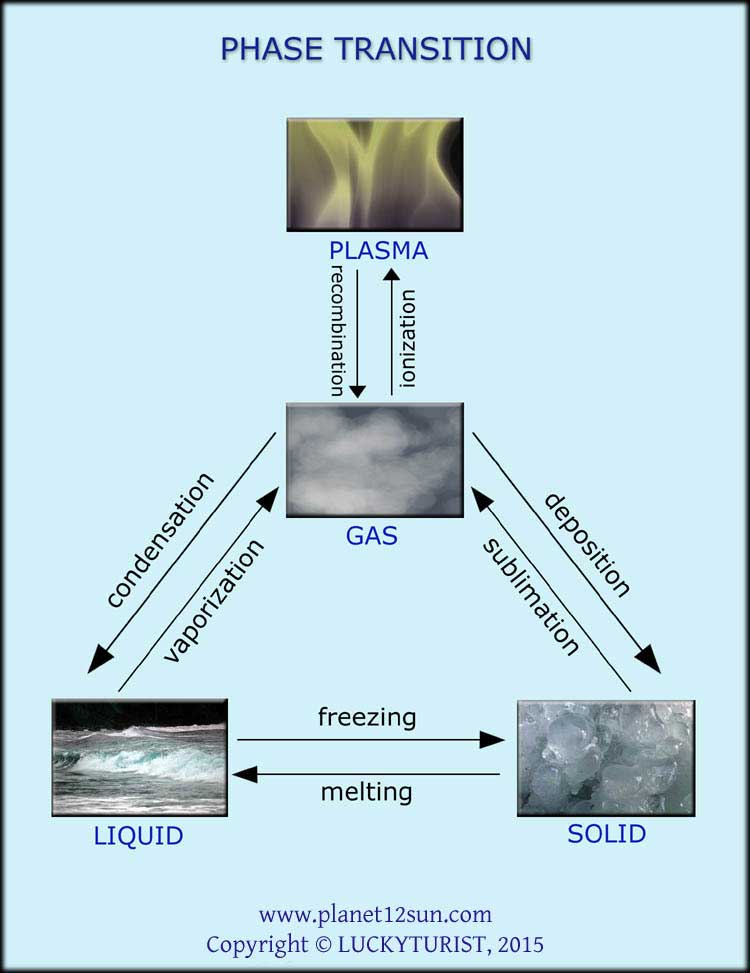
Phase Transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another one by heat transfer. The term is most commonly used to describe transitions between solid, liquid and gaseous states of matter, and plasma.